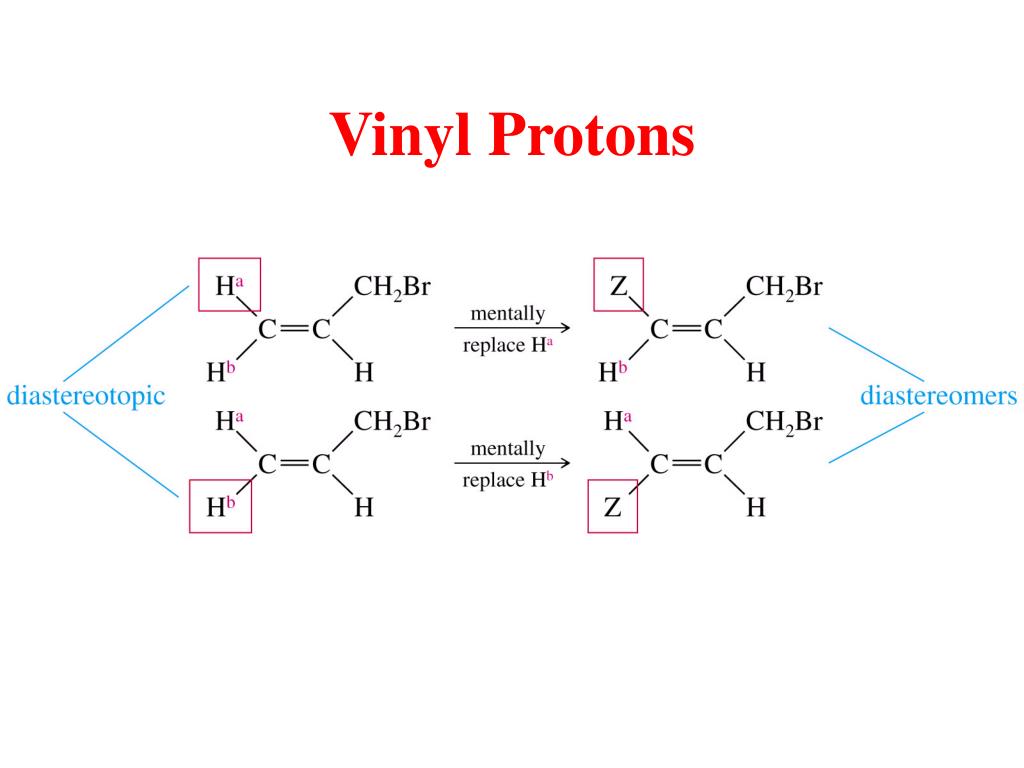
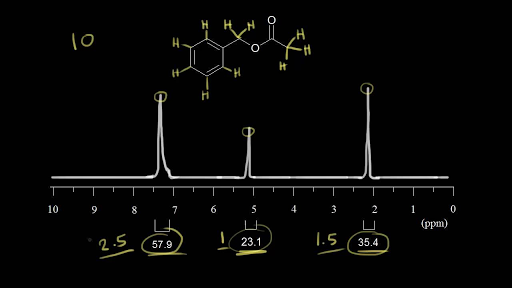
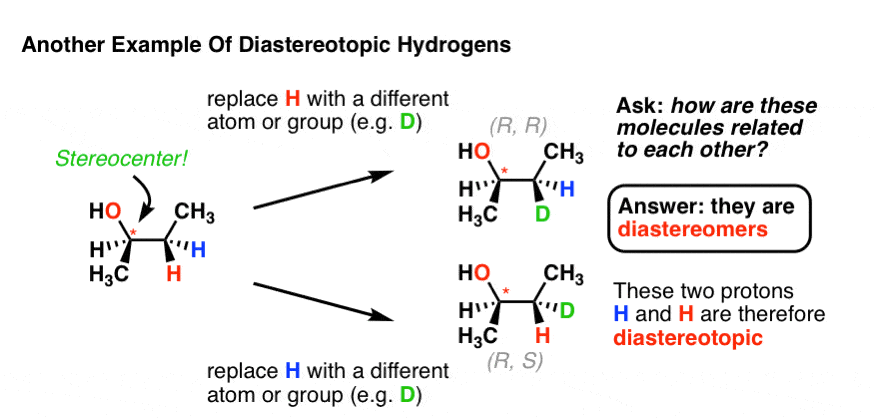
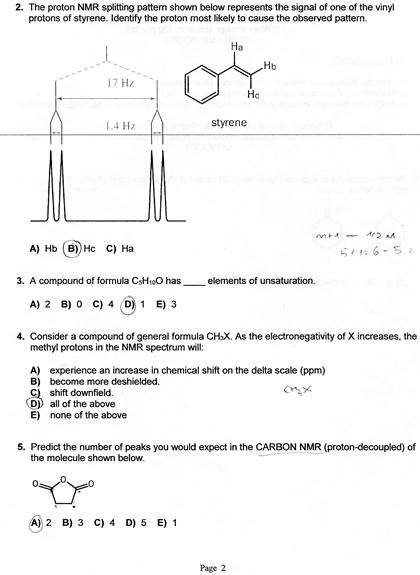
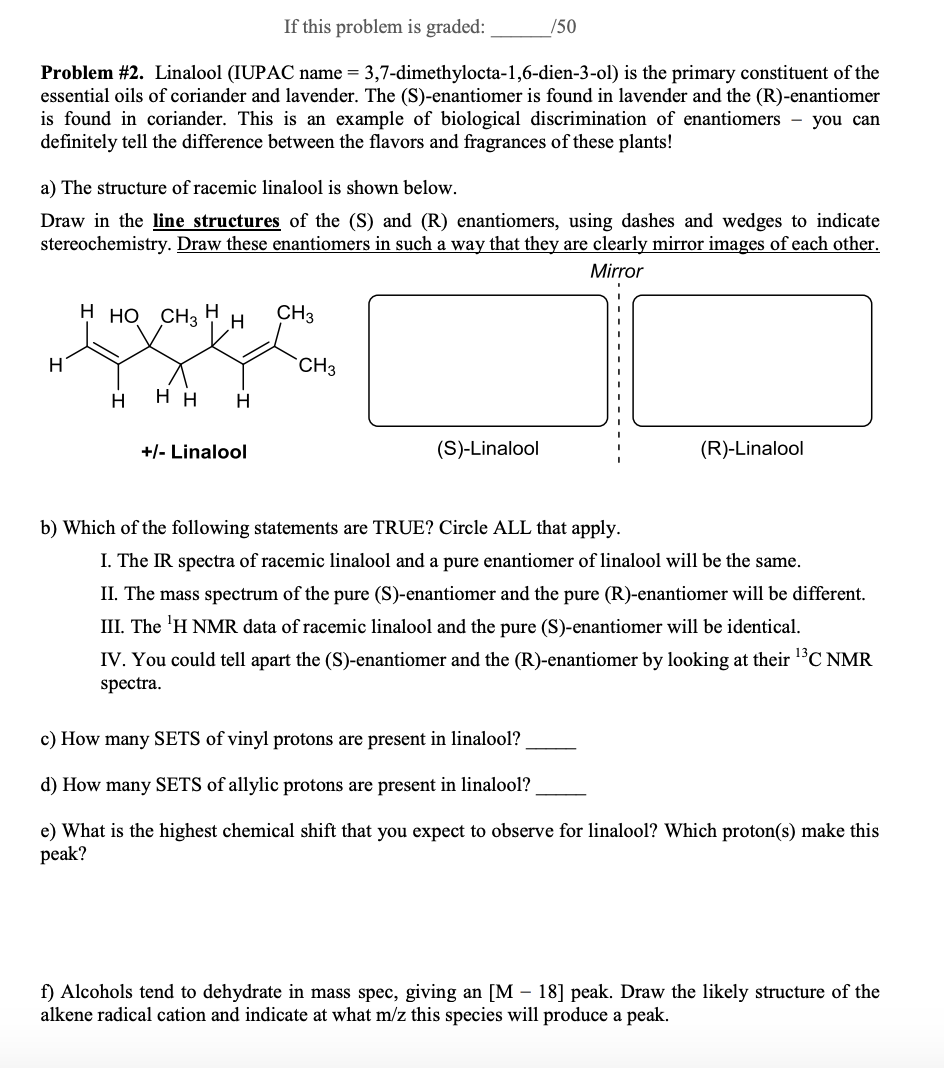
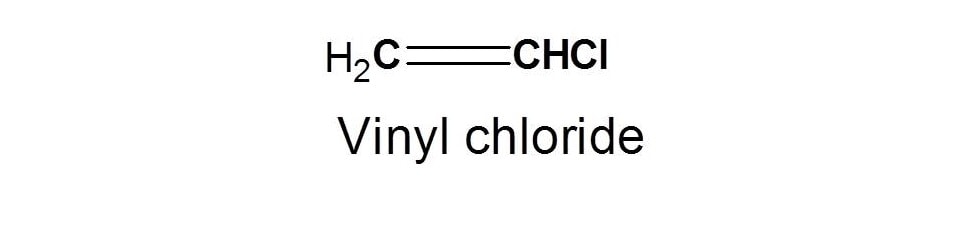
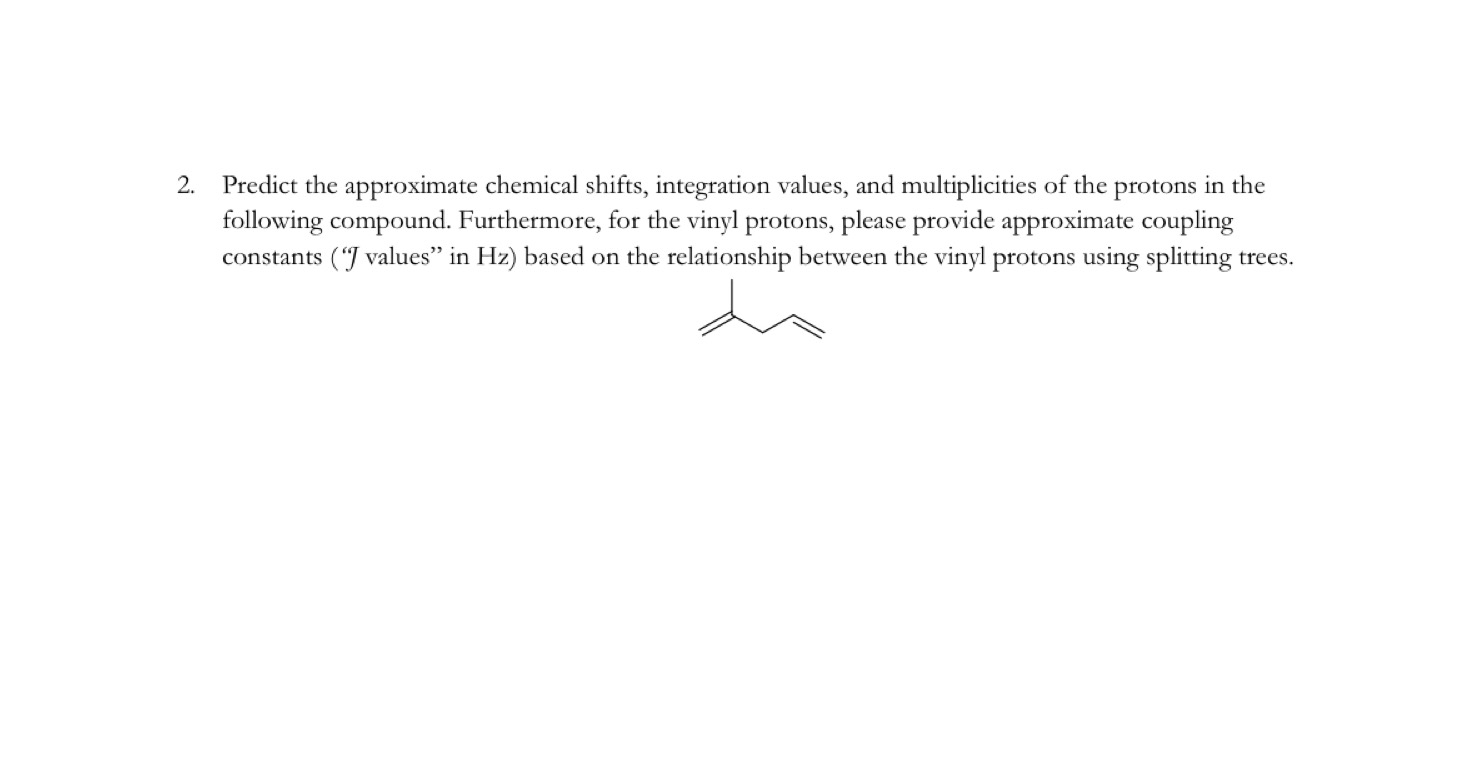
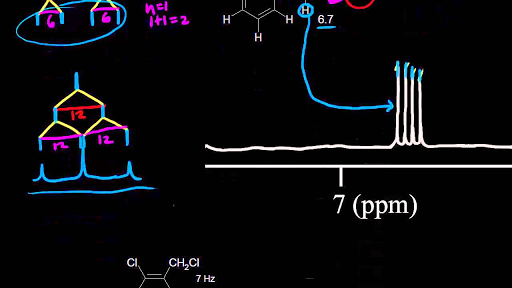
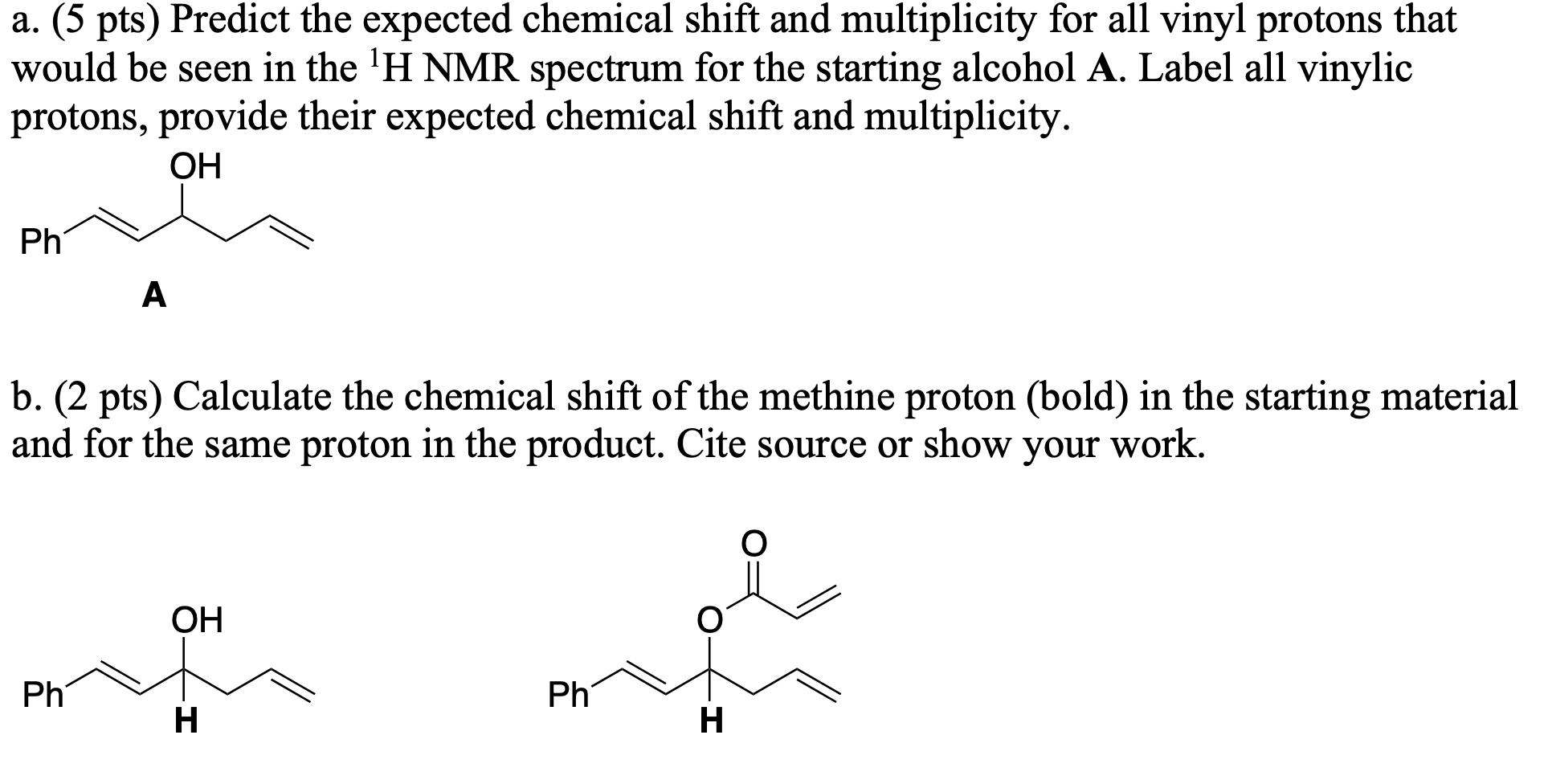
Chemical Shift Of Vinylic Protons
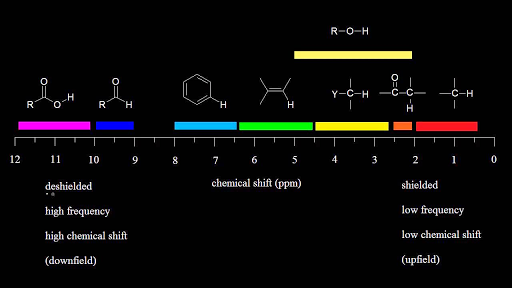
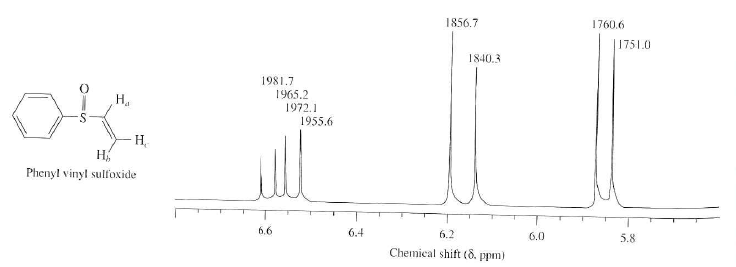
Notice that the proton closest to the carbonyl group is at a higher chemical shift than the proton in cyclohexene 6 05 ppm for cyclohexenone vs.
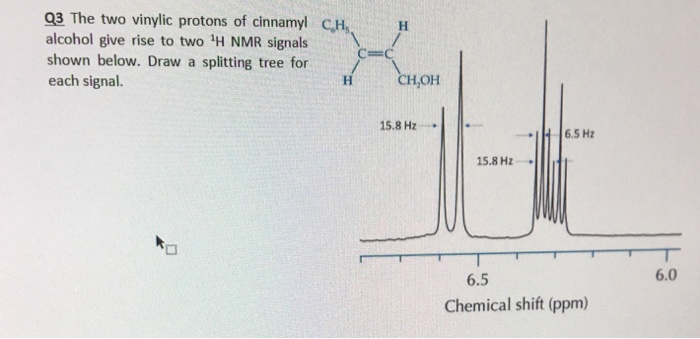
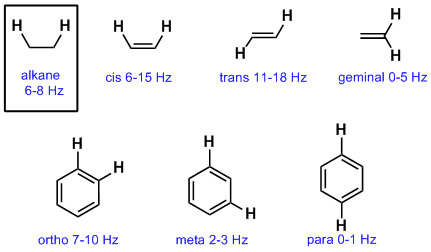
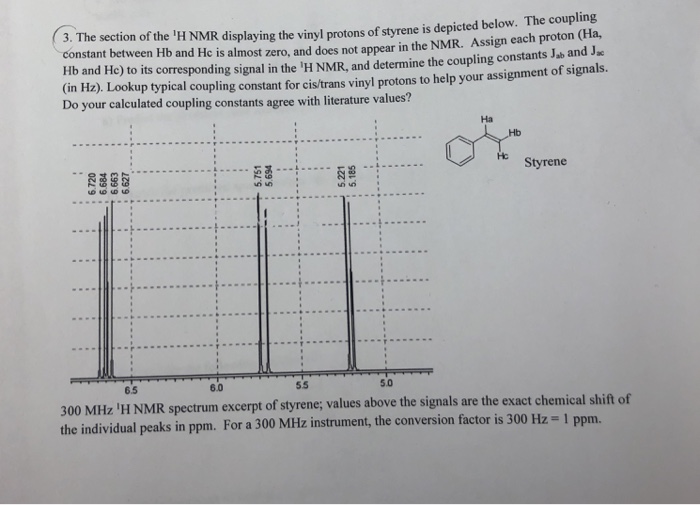
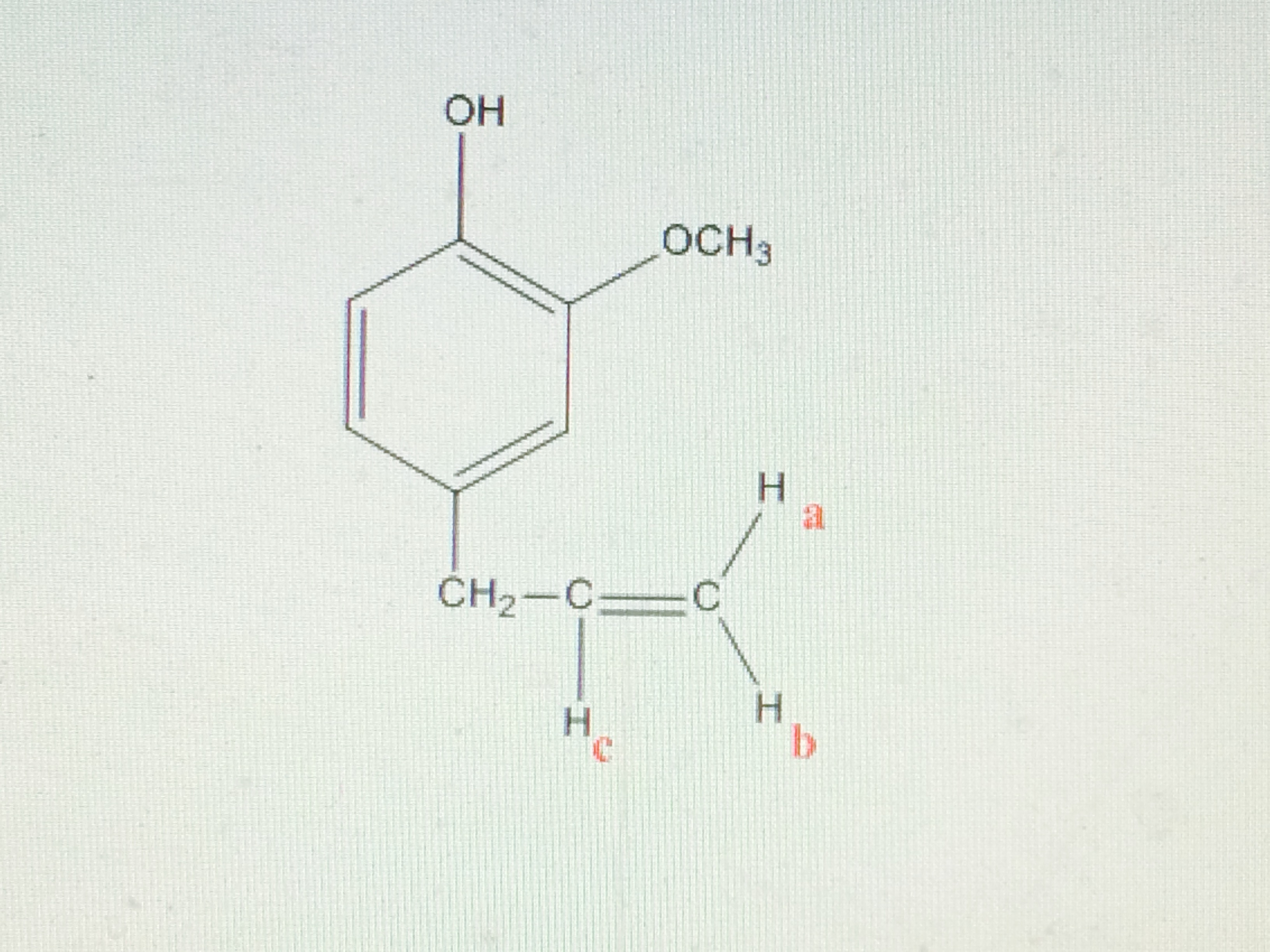
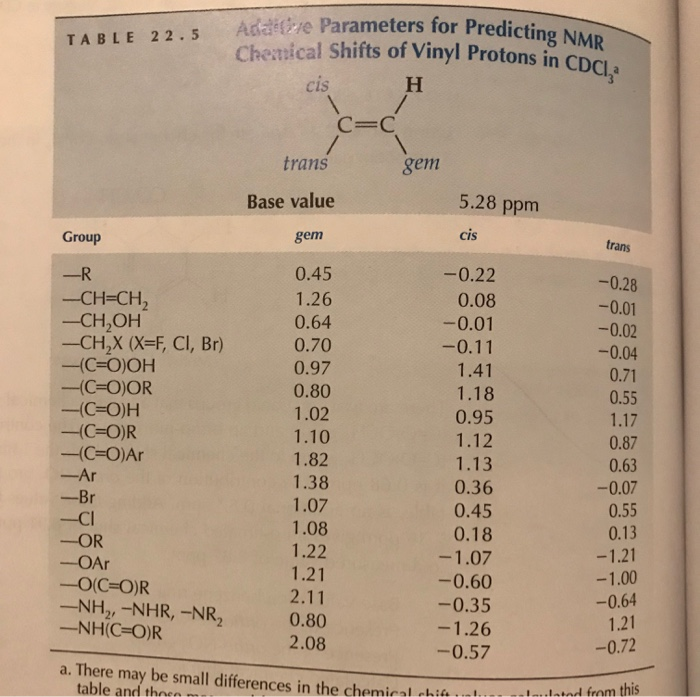
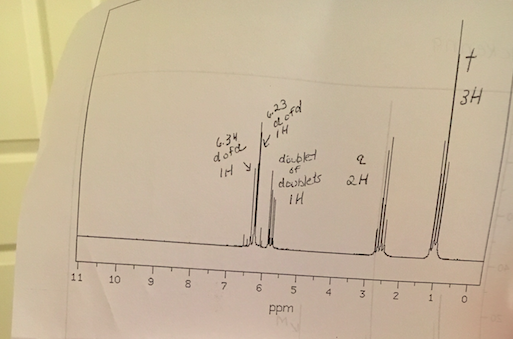
Chemical shift of vinylic protons. We know that a proton alpha to a carbonyl group is pulled downfield. Those bonded to carbon atoms which are in turn bonded to a highly electronegative element. The chemical shift of a vinylic proton is an average over all orientations of the molecule. Splitting between vinylic protons in alkenes depends strongly on the geometrical relation ship of the coupled protons.
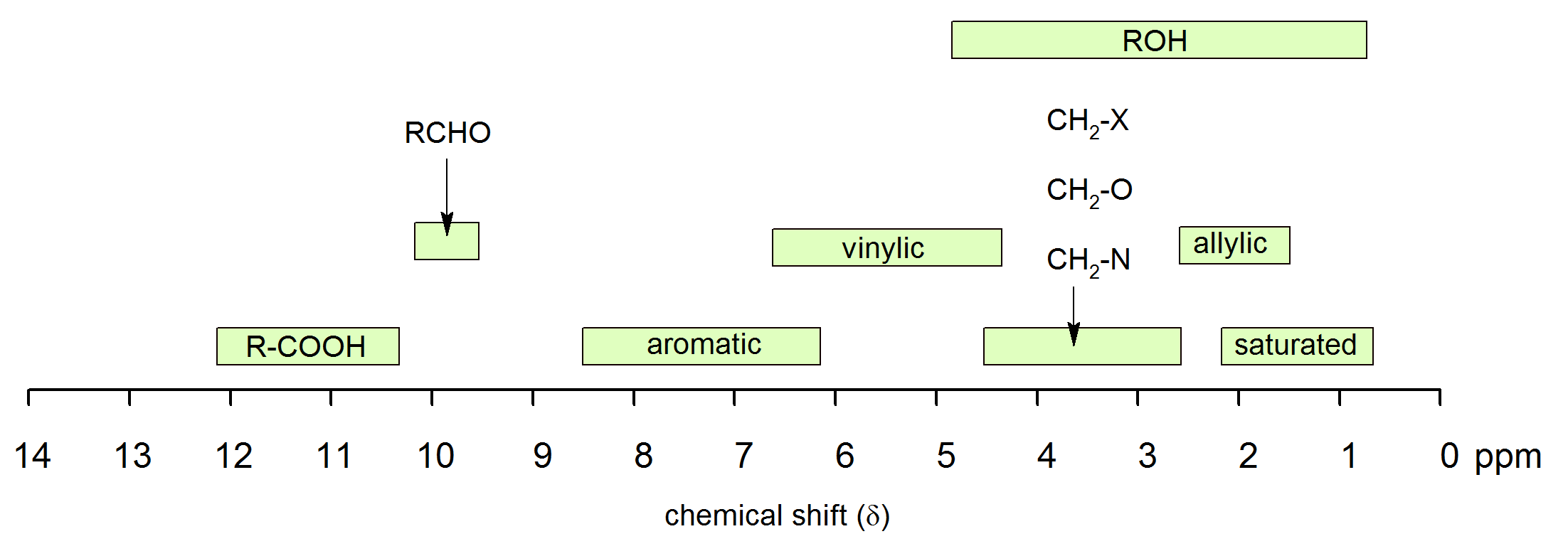
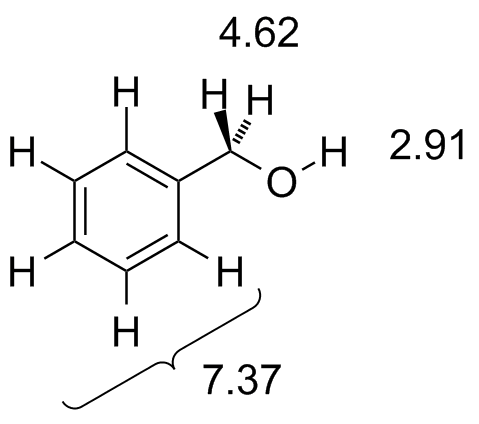
The chemical shift of protons connected to heteroatoms the second group of protons giving signal in this region is the ones bonded to heteroatoms such as oxygen and nitrogen. Those bonded to carbons which are next to unsaturated centres. It is important to understand trend of chemical shift in terms of nmr interpretation. Table of characteristic proton nmr shifts type of proton type of compound chemical shift range ppm rch 3 1 aliphatic 0 9 r 2 ch 2 2 aliphatic 1 3 r 3 ch 3 aliphatic 1 5 c c h vinylic 4 6 5 9 c c h vinylic conjugated 5 5 7 5 c c h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c ch 3 allylic 1 7 hc f fluorides 4 4 5 hc cl chlorides 3 4.
This is not surprising since the proton is not only vinylic but it is also alpha to a carbonyl group. Characteristic proton chemical shiftstype of protonstructurechemical shift ppmcyclopropanec3h60 2primaryr ch30 9secondaryr2 ch21 3tertiaryr3 c h1 5vinylicc c h4 6 5 9acetylenictriple proton nmr chemical shifts california state university stanislaus. Table of characteristic proton nmr chemical shifts. However this particular orientation makes such a large contribution that it dominates the chemical shift.
Electronegative groups move to the down field left. State the approximate chemical shift δ for the following types of protons. The electrons are said to shield the proton from the applied magnetic field and this means that less energy is necessary to excite the proton from one spin state to another and therefore its chemical shift occurs at lower frequency than it would otherwise. The proton nmr chemical shift is affect by nearness to electronegative atoms o n halogen and unsaturated groups c c c o aromatic.
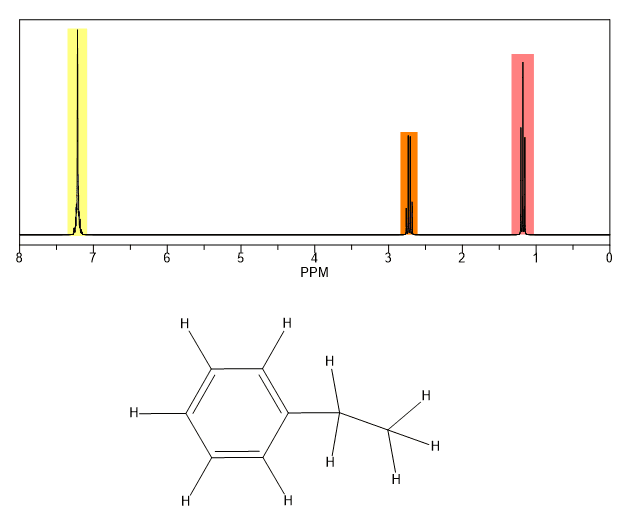
These groups are not aromatic and thus do not generate ring currents as does benzene but the pelectrons circulate in such a way as to generate a magnetic field that adds to b 0 in the regions of space occupied by the protons. Those bonded to carbons which are part of a saturated system. C h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c c h 3 allylic 1 7 h c f fluorides 4 4 5 h.